-
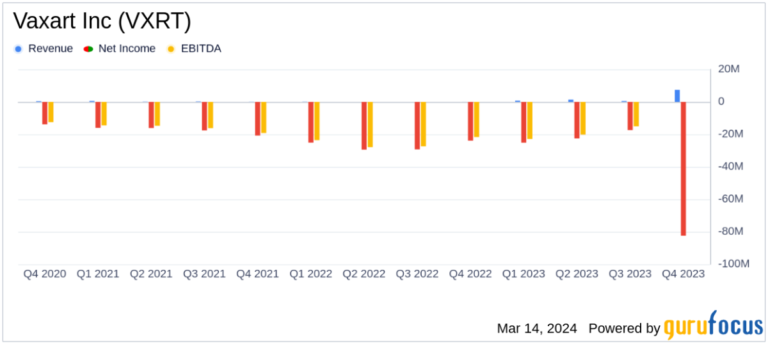
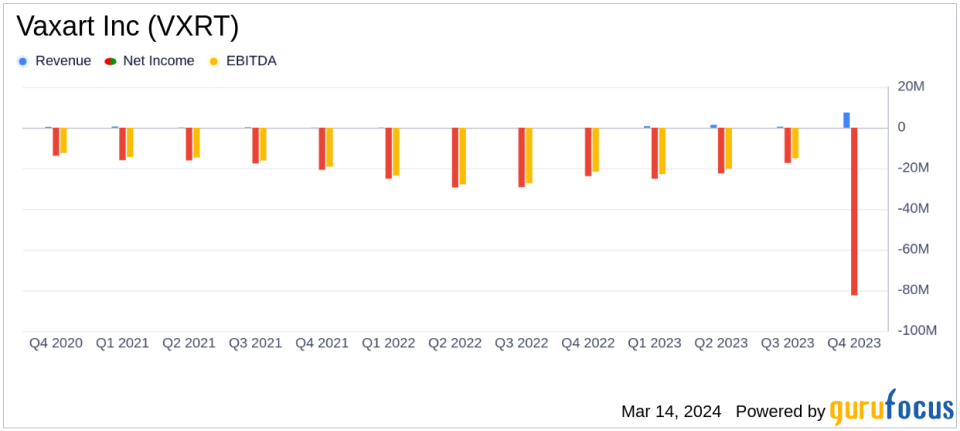
revenue: Reported annual revenue of $7.379 million in 2023.
-
net loss:Net loss widened to $82.465 million, or $0.57 per share.
-
Research and development expenses: Research and development expenses decreased to $68,142,000 from $81,054,000 in the previous year.
-
General and administrative expenses: General and administrative expenses decreased from $29,386,000 in 2022 to $22,584,000.
-
cash position: Total cash, cash equivalents and restricted cash was $34,755,000, down from $46,013,000 at year-end 2022.
-
new leadership: Steven Lo has been appointed president, CEO, and director.
-
Operation progress: Preparations are underway for a Phase 2B trial of an oral COVID-19 vaccine, with data from a Phase 1 norovirus trial expected to be available in mid-2024.
March 14, 2024 Vaxart Inc (NASDAQ:VXRT), a clinical-stage biotechnology company, announced an 8-K filing detailing its financial results for the year ended December 31, 2023. The company is known for developing oral recombinant technology. The Vaccines business' net loss was $82,465,000, or $0.57 per share, compared to a net loss of $107,758,000, or $0.84 per share, in the prior-year period. Despite the net loss, the company made significant progress in its vaccine development program, including preparation for a Phase 2B study of its oral COVID-19 vaccine.
Financial performance and company development
Vaxart Inc (NASDAQ:VXRT) reported that research and development expenses will decrease from $81,054,000 in 2022 to $68,142,000 in 2023, reflecting the company's efforts to streamline its vaccine development program. General and administrative expenses also decreased from $29,386,000 the previous year to $22,584,000, indicating an increase in operational efficiency.
The company's cash reserves decreased to $34,755,000 from $46,013,000 at the end of 2022, highlighting continued investment in clinical programs. Nevertheless, Vaxart continues to make progress, with Steven Lo joining as our new president, CEO, and board member, bringing extensive experience as a biopharmaceutical leader to the team.

Strategic focus and future outlook
Vaxart's focus on developing oral vaccine platforms is further strengthened by the recent BARDA Project NextGen contract win, supporting the company's differentiated approach to vaccine delivery. The oral pill vaccine being developed by Vaxart could offer several advantages over traditional injections, including ease of administration and the potential to vaccinate more people quickly and painlessly.
Looking ahead, Vaxart expects to have topline data from its Phase 1 norovirus study in breastfeeding mothers by mid-2024. This, along with the upcoming Phase 2b trial of its oral COVID-19 vaccine, will position the company at the forefront of innovation in the vaccine industry.
The company's commitment to revolutionizing the way vaccinations are administered through its unique delivery platform is evident in its extensive patent applications and ongoing development programs for vaccines against coronavirus, norovirus, influenza and HPV.
For value investors and potential GuruFocus.com members interested in the biotech space, Vaxart Inc's (NASDAQ:VXRT) approach to vaccine development and future growth potential makes it an interesting company to consider for further research and investment. It may be an opportunity.
For a detailed analysis of Vaxart Inc (NASDAQ:VXRT)'s financial results and strategic initiatives, visit GuruFocus.com.
For more information, please see the full 8-K earnings release from Vaxart Inc here.
This article first appeared on GuruFocus.