New York's attorney general on Thursday called on the Food and Drug Administration to “take immediate action” and renew warnings to doctors and patients about Singulair's dangerous effects on children, adding to current warnings about the drug's psychiatric side effects. said it was not enough.
Attorney General Letitia James also asked federal officials in a letter to consider refraining from prescribing the asthma and allergy drug Singulair to children.
Thousands of patients and parents have complained to the FDA that symptoms of anxiety, agitation, hallucinations and other psychiatric problems are linked to the drug, also known as generic montelukast. Those reports, coupled with an emotional FDA hearing in 2019 and cases cited in the medical literature, led the FDA in 2020 to order the most severe warning on the drug's instructions.
But a New York Times investigation found that people continued to report not knowing about possible side effects, including suicide or suicide attempts, when they took the drug or gave it to their children. .
Citing the Times article, Ms. James called on the FDA to “introduce new and more stringent safety regulations for this drug,” especially for children.
“Parents and guardians have the right to be fully informed about the potential side effects of medications when making choices about their child's health,” James said in a statement Thursday. “The risks associated with the use of Singulair are too dire to be approached without clear warning.”
Asked for comment, FDA spokeswoman Chanapa Tantivanchachai said Thursday that the agency would respond directly to James.
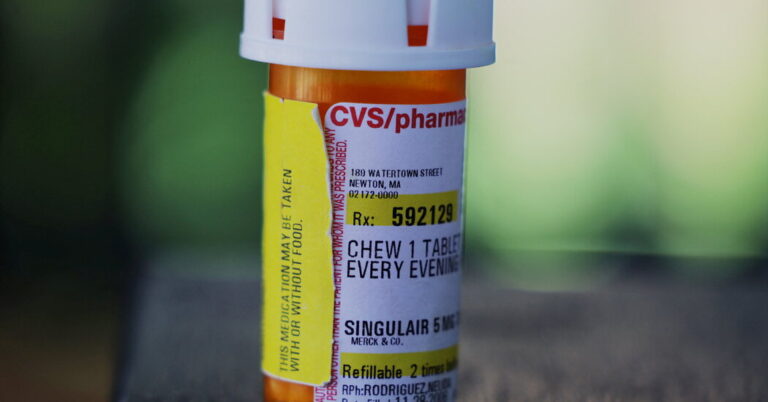
This drug was an early blockbuster for Merck. Now available as a generic drug, the drug continues to be a go-to for doctors, especially because it allows children to take a once-daily chewable pill rather than using an inhaler. It is not a steroid, which is another reason why it is considered an option for asthma patients.
More than 12 million people wrote a prescription for the drug in 2022, according to data provided to the Times by medical data firm Comodo Health.
Merck continues to defend the drug in court, but earlier submitted comments to generic drug maker Organon, which said the drug's risks have been communicated to patients and health care providers. .
In the face of years of criticism for the drug's availability despite the risks, the FDA said it acted appropriately in response to concerns about the drug. The agency continues to study and monitor the drug, but said studies large enough to pinpoint rare events associated with the drug, such as suicide, are not feasible.
James' letter outlines further steps the FDA could take, including communicating the safety of new drugs to doctors, pharmacists, and other health care providers. She called for further review to ensure the drug still offers more benefits than risks for children.
Thomas Moore, a drug safety expert who has tracked reports of montelukast's psychological effects for years, urged the FDA to investigate drug companies to determine whether warnings were reaching patients. He said that it is known that
“This means that all parents of children taking this drug should be on the lookout for unexpected behavioral changes and consider this a possibility,” said Moore of the Johns Hopkins University Center for Drug Safety and Effectiveness. We emphasize that this should be taken into consideration as a
Kamie Panney, administrator of a Facebook group for people who say they have been affected by the drug, said she was pleased to see James taking action and calling for a deeper investigation. Told. She believed her son was suffering from side effects in 2017. It was her arduous task to console her parents who found her group.
“The time has come,” said Panny. “My goal one day is to not have to do this anymore.”